




|
|---|
Like iron and copper, aluminum is also an essential metal. However, unlike those two, its compounds do not have a characteristic color; in fact, aluminum compounds are almost universally colorless, and are relatively transparent in most media. While this makes them useless as pigments on their own, it also allows them to become a substrate for colorful dyes.
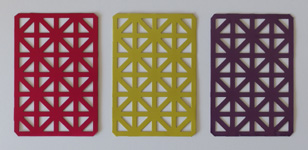
Many natural dyes have an affinity for polyvalent metals, which act as a mordant (from the French word for "biting") in that they allow the dye to bind to a substrate. Of all metals, these dyes are most effectively used in combination with aluminum, due to its colorless nature and chemical stability. The method here consists simply of creating an insoluble aluminum compound within the substrate to be dyed, followed by immersing the substrate in a dye solution so that its color can be absorbed. In practice I have been able to achieve this on silk, aluminum, and chalk, in order to color the surfaces of objects and to create dye-based pigments. Examples of these can be seen at the top of the page, and the methods and ingredients to create them are described below. Note that the ratio of ingredients is often based on a given weight of fiber, commonly abbreviated "WOF"; in cases where fiber is not being dyed, an arbitrary value should be used to complete the calculations.



|
|---|
The first material I chose to dye was silk; protein fibers are the usual substrate for most natural dyes, so existing methods were well-documented. The dyeing process consists of immersing and stirring the fibers in three chemical baths, rinsing between each step, and using enough water in each bath to cover the fibers completely (100x WOF). The first is an alkaline cleaning bath, which in my case used sodium carbonate at 10% WOF. The next bath contains the mordant, for which I used aluminum sulfate at 10% WOF. Last is the dye bath, the concentration of which depends on the dye being used; in the pictures above, I used brazilwood at 50% WOF to dye the silk a bright red. All baths were used at around 50-75°C to avoid burns, with each immersion taking half an hour. Extracting the dye from its raw materials (and subsequently filtering out the solids) took an hour, however this can be achieved during the cleaning and mordanting stages, with the entire process taking an hour and a half.


|
|---|
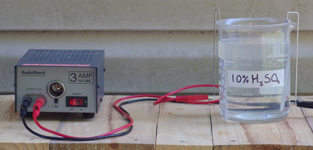
The next material which can be dyed is aluminum itself. The process is identical to that for dyeing silk, with the exception that the mordanting bath is replaced by an anodizing bath consisting of roughly 10% sulfuric acid at room temperature. In this bath, the workpiece is attached to the positive terminal of a 12-volt power supply, with a piece of scrap aluminum connected to the negative terminal, using aluminum wire for any submerged connections to avoid contamination from other metals. The anodizing process should be conducted outdoors (due to the irritating vapors) and takes half an hour, resulting in a ceramic-like coating of aluminum oxide which readily absorbs dye. In the pictures above, I used osage at 30% WOF to dye a decorative piece of sheet aluminum a bright yellow. Unfortunately since aluminum has far less surface area than silk, most of the dye remains unused; however, since less-concentrated dye baths result in slow and incomplete dyeing, the best option is to simply repurpose or reuse the remaining dye. Likewise, the sulfuric acid can be reused or repurposed by saturating it with scrap aluminum, which results in a roughly 25% stock solution of aluminum sulfate, useful as a mordant for the other processes.



|
|---|
Finally, I chose to produce a dye-based pigment by reacting aluminum sulfate with calcium carbonate (chalk). This produces a composite pigment consisting of aluminum hydroxide, calcium sulfate, and a small amount of leftover chalk, all of which are relatively transparent in oil paint. This pigment could be prepared on its own and subsequently dyed, but a more effective method is to prepare a dye bath (identical to those used earlier), then stir in the aluminum sulfate followed by chalk in the ratio described above. Preparing and filtering the dye bath takes an hour, and the reaction between the mordant and the chalk should be given half an hour (at 50-75°C) to complete. The resulting slurry should have little to no dye remaining in the liquid portion, and can then be filtered to recover the pigment. In the pictures above, I used logwood at 10% WOF to produce an intense violet pigment, which I then used alongside pigments made from the previous two dyes to make the oil paints seen at the top of the page.
Using the procedures described above, I believe I have made it possible to produce colorful objects and pigments using nearly any dye, using a fixed set of ratios and ingredients. However, this article is by no means comprehensive. Dyed colors can of course be varied by using more or less dye, but also by changing the pH of the dye bath (such as with vinegar or ammonia), by changing the mordant composition (such as with iron or copper sulfate), or by simply mixing multiple dyes. Exhaustively documenting these variations and combinations would greatly lengthen this article however, so I will leave those experiments as an exercise to the reader. At this point I am satisfied with this method, and I look forward to using it in projects to come.