






|
|---|
During my experiments with making paper sensitive to light, I noticed that the paper I treated was also sensitive to electricity; two probes attached to a battery would turn the paper blue if placed in close proximity to each other, as long as the paper was still damp. I found this interesting, and decided it merited further investigation; specifically, I wanted to develop a sensitizing solution which was stable to light and air, and to find a type of paper which would saturate easily while remaining strong enough to write on.
My first course of action was to create the sensitizing solution, as I believed this would be the most difficult problem to solve, and I had found that coffee filters were suitable as test papers for the time being. Since this solution would have to be highly conductive as well as non-drying, I decided to base it on calcium chloride, which is a deliquescent salt; under normal conditions, it absorbs enough moisture from the air to dissolve itself, and so provides a stable level of conductivity within a saturated sheet of paper. The solution also needed to contain potassium ferricyanide and a source of iron; however since I was unable to find any common iron compounds which were stable to both light and air, I decided to use a consumable iron stylus instead. When positively charged, the iron dissolves into the saturated paper, where it reacts with the ferricyanide ions to produce prussian blue. After considerable optimization of this sensitizing solution, I eventually settled on the formula below:
|
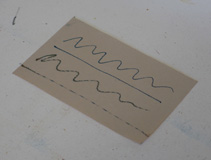
The next step was to find the optimal paper for use with this solution; the paper needed to be thin and highly absorbent, but also durable enough for sustained writing. Filter paper was suitable in the first two regards, but was easily damaged by the friction of the stylus due to its rough surface. I eventually discovered that newsprint paper had the necessary qualities; it absorbs water readily, retains considerable strength when wet, and provides a slick writing surface which shows surprising wear resistance. If a piece of newsprint is soaked in a tray of the above solution and then laid onto a brass backing plate, it can be written on in blue pigment using a pointed iron rod and a source of electricity. Direct current produces a solid blue line, while alternating current produces a dashed line if the stylus is moved quickly enough. The paper can then be rinsed and developed much like a cyanotype. These results can be seen at the top of the page.
While this paper may appear to be simply an interesting demonstration, in actuality it is highly practical. As shown above, it is capable of responding rapidly to changes in current; if the stylus were arranged to move automatically, it could easily be used to recieve images transmitted over a wire. I intend to investigate this in great detail in the future, as I believe this is of critical importance in the simplification of technology. After all, what is a webpage, but an image sent over a wire? This type of work will occur later, however; for now, I am not yet through with investigating prussian blue.