






|
|---|
Prussian blue, along with having a very controllable synthesis, is also controllable in pigment form. Under reducing conditions it becomes colorless, and upon oxidation it returns to its original blue state. If a thin layer of prussian blue is formed on a piece of conductive glass, and this glass is placed in an electrolyte containing potassium ions, this phenomenon can be utilized to create an electrochromic display. Since conductive glass is fairly easy to make, I decided to build an electrochromic panel as the final entry in my series on the chemistry of prussian blue.
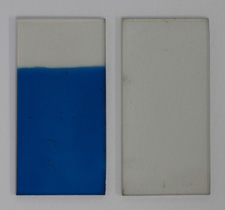
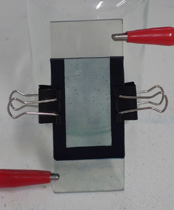
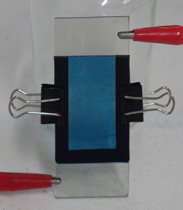
First, I made two slides of conductive glass by my usual method, achieving a sheet resistance of five ohms per square. I then made a solution containing 0.01M potassium ferricyanide and 0.01M ferric chloride in 0.01M hydrochloric acid; this solution is fairly unstable (decomposing to prussian green in a few hours), but serves as a plating bath to deposit prussian blue on the glass. After plating for a few minutes at a current density of around 30µA per square centimeter using a graphite anode, I was left with a suitably thick and adherent layer of pigment on one of the glass slides. After rinsing this slide, I then clipped the two slides face-to-face, separated by a gasket made from sheet rubber, and filled the gap with a 1.0M solution of potassium chloride. These steps can be seen at the top of the page.
In this configuration, the color of the panel can be controlled simply with a single 1.5V AA cell. With the prussian blue made negative, it is reduced to prussian white, which is transparent. If the cell is reversed and the prussian blue is made positive, it becomes blue again. At this voltage the transition is fairly slow (around five seconds), but can be repeated indefinitely. With two cells in series (for a battery voltage of 3.0V), the transition takes only a second, but the pigment can easily be broken down by continued oxidation, at which point the panel becomes unresponsive. Fortunately, the pigment can be cleared from the glass by first soaking it in an alkali (to decompose it to ferric hydroxide), then in oxalic acid to remove the otherwise-insoluble iron. These processes are the same as the developing stages in my cyanotype method, and the chemistry is identical. By this method the glass can be reused if it ever becomes "broken" during experimentation, and in fact the two pictures at the top right feature glass which has been re-plated in this manner.
Panels of this sort could be used as-is for electronically-tinted windows, which is their most common commercial use. However, if the glass or the pigment were etched into segments or symbols, this could be utilized as a low-power digital display. Even at three volts, the panel only requires a few milliamps to change color, and the color is stable until a reverse current is applied. The total transparency of the panel also allows for the possibility of transparent displays, such as within windows or mirrors. However, these applications are currently beyond the scope of this website, and are left as an exercise to the reader. At this point, I believe I have explored the chemistry of prussian blue to my own satisfaction, and will now continue on to other projects.