During my experimentation with solar cells I have made a significant quantity of conductive glass, and will need to continue making it in the future. To make the process less hazardous, I have decided to eliminate fluorine from the precursor solution by using antimony (Sb) as a dopant instead. Antimony is less toxic than fluorine, and due to its low volatility a smaller amount is needed for doping. Furthermore, as a matter of convenience, the elimination of fluorine allows the spray solution to be prepared and stored in glass containers.
Antimony-doped tin oxide is one of the oldest types of highly-conductive glass coatings, so the optimal doping level is well known to be 2% by molarity. That is to say, a 1M solution would contain 0.98M of tin and 0.02M of antimony. To make this, I decided to prepare a stock solution containing antimony, into which I would dissolve stannous chloride immediately before making a piece of conductive glass. I began by dissolving 1.31g of antimony (III) oxide into 112.5mL of 20% hydrochloric acid, which I then diluted to 450mL with distilled water. The excess acid (5%) is necessary to keep the antimony dissolved, similar to how stannous chloride behaves in solution.
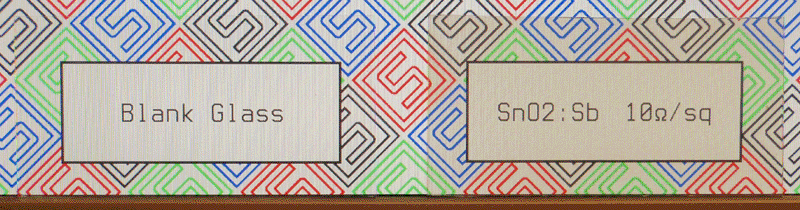
At this point, I poured 30mL of the above solution into an airbrush bottle and added 6.64g of stannous chloride. I then heated a number of pieces of glass to between 400°C and 500°C on my hot plate and sprayed them with varying amounts of the solution. Despite repeating this process a number of times, the results were consistently mediocre, achieving a minimum sheet resistance of 20 ohms per square and generally having poor transparency. I then decided to change the doping level to 1% by using only 15mL of the antimony solution, which I then diluted to 30mL with distilled water and enough acid to redissolve any precipitates. I again added 6.64g of stannous chloride, and prepared a 4-inch by 4-inch piece of glass in the usual manner using 15mL of the new solution. The results can be seen at the top of the page; the glass has been cut in half to better fit the screen.
The new glass is comparable in every aspect to the fluorine-doped glass I had been preparing earlier; the transparency, conductivity, and solution usage are nearly identical. Since the required doping level was different than what I expected, however, I will attempt to find the optimal value as I make more conductive glass for my solar cells.