
Wood and iron are a common and useful pair of materials, which interact well together and can form the basis of a wide variety of projects. As such, it is important to be able to finish both materials in a way that shares a common aesthetic. For glossy, decorative work, my varnish and enamel work well together, but for flat and functional objects, I needed a finish for iron to match the "stain, oil, and wax" method used in the previous article. There are a number of common methods for producing a flat, black surface on iron or steel components, and I investigated them thoroughly. Unfortunately, each of them had at least one aspect that precluded their use. These methods and limitations can be seen in the table below:
| Method | Pigment | Limitations |
|---|---|---|
| Heat-Bluing | Iron Oxide | Components become annealed and distorted, difficult to achieve on large parts |
| Rust-Bluing | Iron Oxide | Time-consuming, difficult to achieve on internal surfaces |
| Cold-Bluing | Copper Selenide | Solution contains hazardous quantities of selenium (poisonous) |
| Parkerizing | Manganese Phosphate | Solution contains hazardous quantities of manganese (neurotoxic) |
Of these options, however, the Parker process (parkerizing) was the most appealing. This consists of immersing iron or steel components in a hot solution of manganese phosphate for approximately 15 minutes. The result is a hard and rustproof black coating that is porous enough to hold oil. Fortunately, this is actually an extension of the earlier Coslett process, in which (non-toxic) iron phosphate is used to the same effect. This produces a soft phosphate coating of moderate rust resistance, but for my purposes this is perfectly adequate.
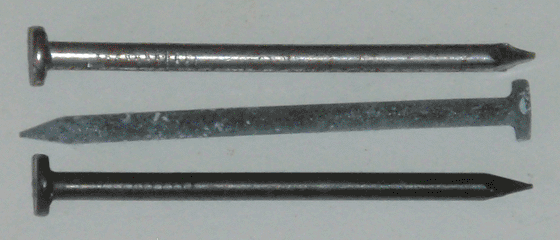
Preparing the iron phosphate solution is simple, and consists of dissolving any source of iron (in my case, steel wool) into a simmering 1% solution (by weight) of phosphoric acid in water, until the bubbling stops and a fluffy white precipitate (insoluble iron phosphate) begins to form. At this point the iron source will have gained a black phosphate coating, and any new piece of iron added to the solution will receive the same in about an hour. The piece can then be removed and rinsed, and when dry, a thin coat of linseed oil can be rubbed on to darken the color and improve the rust resistance. The results of these steps can be seen at the top of the page, as applied to ordinary steel nails.
Due to the widespread availability of phosphoric acid as a metal primer and concrete etchant, this method is cheap and easily accessible. The only limitation is the size of the immersion tank; however, due to the fact that the phosphating reaction is self-limiting, cast iron pots or welded steel containers can be used to coat large objects without risk of corrosion. Furthermore, the solution can be reused until it becomes depleted, at which point more phosphoric acid and iron can be added to bring it back to its original concentration. Finally, although insoluble phosphate sludge will eventually accumulate, this can be removed by filtering the solution back into a storage bottle between uses.
Although I look forward to using this method in future projects, revisiting my collection of coatings and finishes has revealed the potential for some improvements, as well as some applications where my collection is currently lacking. I will likely take some time to address these issues before returning to my mechanical projects, so that I can be certain I am using the best possible materials in the things I make.