
Soldering is the most versatile and precise method of permanently joining metal components, and I have used it extensively in the past. Despite this, I have never investigated it in detail, and have generally relied on low-cost plumbing solder and its associated paste flux to accomplish the task at hand. This has often been inadequate, so I decided to investigate soldering as a whole in more detail, before I continue on to future projects that will require it.
To begin, I decided to investigate solder alloys; these are often specified as a weight ratio of two or more metals, having either a melting range or a melting point. Those with a melting range are slush-like within this range; this allows for the filling of larger gaps, but also presents the risk of a cold joint if the solder is disturbed as it cools. Those with a single melting point are eutectic alloys; these are more fluid and have a stronger capillary action, as well as having little potential for cold joints due to the absence of a slush phase. Each unique combination of metals generally only has one ratio which is eutectic, and some of those which are useful for soldering can be seen below.
| Composition | Eutectic Ratio | Melting Point |
|---|---|---|
| Bismuth-Tin-Lead | 52/16/32 | 95°C |
| Bismuth-Tin | 58/42 | 138°C |
| Tin-Lead | 63/37 | 183°C |
| Tin-Zinc | 91/9 | 199°C |
| Tin-Silver | 96/4 | 221°C |
| Silver-Copper | 72/28 | 779°C |
While these alloys could all be made in the home workshop if necessary, they are readily available for little more than the cost of their component metals. Having purchased a number of the above compositions to experiment with, I then moved on to investigating fluxes. As their name suggests, fluxes promote the flow of solder over the surfaces to be joined. Since metal oxides repel liquid metal, this is typically accomplished by removing these oxides chemically. As such, the components of the flux strongly depend on the type of oxide to be removed, as well as the temperature at which this removal must occur. Unlike solders, commercial fluxes typically conceal the true ratio of their components, which themselves are often unnecessarily hazardous. For this reason, I decided to develop my own.
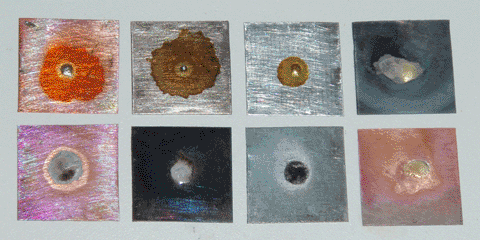
I began by making a rosin-based flux. Rosin is the oldest low-temperature flux in common use, and can simply be used in its solid form for some applications. This is inconvenient, however, so I made a brushable flux by dissolving pine rosin into 190 proof grain alcohol (95% ethanol) in approximately a 1:1 ratio by weight. This allows the rosin to flow into small gaps at room temperature, leaving behind a thin film as the alcohol evaporates. At soldering temperatures the rosin melts, and due to its acidic nature it reacts with metal oxides to form metal resinates, which dissolve into the rosin itself. I then tested this flux on small pieces of copper, steel, and zinc (galvanized steel). The pieces were sanded and cleaned with acetone, then a drop of flux and a clipping of tin-lead solder were placed on the surface. I then placed all three pieces on a hot plate and quickly raised the temperature to melt the solder. The results can be seen below.


The rosin flux performed well on copper, adequately on zinc, and poorly on steel, reflecting the increasing stability of their respective metal oxides. It appeared to work best at a temperature of around 250°C, beyond which the rosin decomposed and no longer functioned as a flux. It follows, then, that for metals with tenacious oxides or solders requiring higher temperatures, a different flux is necessary; for this purpose, I made a second flux based on zinc chloride.
Zinc chloride has the useful property of dissolving metal oxides when molten (above 290°C), and when coupled with hydrochloric acid as an etchant, it forms a powerful flux. This is most commonly made by dissolving a source of zinc into an excess of acid, and I made a small batch from zinc oxide and 20% (6M) hydrochloric acid in a weight ratio of about 1:5. I then prepared three pieces of the same materials as before and ran an identical test using the hot plate, the results of which can be seen below.


This flux is obviously much more powerful, allowing the solder to wet copper, steel, and zinc equally well; however, it comes at the expense of a higher temperature requirement (roughly 350°C) and an acidic residue which must be washed off. These strengths and limitations result in a flux that is best suited to structural applications, unlike the previous flux which is more suited to electronic work.
For structural components requiring extreme strength or heat resistance, however, an entirely different approach must be taken. Rather than a "soft" (tin-based) solder, a "hard" (silver-based) solder must be used. These have a working temperature higher than the boiling point of zinc chloride, so in general boric acid is used as a flux. I prepared a flux of this type by simply wetting boric acid with 95% ethanol, adding just enough of the alcohol to cover the resulting paste. I then applied this flux to test pieces of copper and steel, along with a small clipping of Safety-Silv 45 (45% silver, 30% copper, 25% zinc) from Harris. Bringing the pieces to a red-orange heat with a propane torch melted the flux and the solder, and in both cases the solder wet the metal surface adequately.


Overall I am highly satisfied with the three fluxes described above, and they appear to be entirely suitable for the work I intend to do. Since I have no intention of soldering stainless steel, I have not included any fluoride compounds, and as a result the fluxes are essentially harmless. Future projects will be a true test of their utility, but for now I see no reason not to make them my first choice for any soldering tasks I encounter.