
Further experimentation with dye-sensitized solar cells yielded little improvement in terms of efficiency. Ultimately, to make a useful cell I would likely have to resort to using commercial titanium dioxide nanoparticles, complex chemical syntheses, or rare and toxic solvents. None of these options fit the theme of this website or the motivations for my work, so I returned to my previous attempts at making thin film solar cells. Earlier, I had spent a great deal of time working with selenium, without much success. I spent comparatively little time on copper oxide, another common semiconductor, so I decided to focus my efforts there.
Copper (I) oxide (Cu2O) can be formed in a number of different ways, but one of the most convenient is by electroplating. Hot alkaline solutions of complexed copper ions will deposit Cu2O on the cathode, if the plating current is kept fairly low. In my case, I used a solution with the following ingredients:
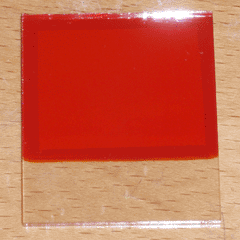
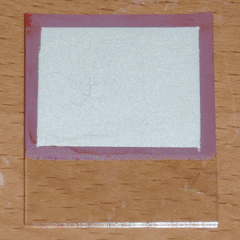
Once the solution was prepared, I initially attempted to deposit copper oxide on my conductive glass at a temperature of around 60°C and a current density of 1mA per square centimeter, using the setup pictured at the top right of the page. After about a half hour, this would produce a semi-transparent red film of Cu2O; however, the resulting film was full of pinholes and was therefore unusable. This was the case no matter how thoroughly the glass was cleaned, so it is likely a product of the tin oxide coating itself. I then decided to use the aluminum-doped zinc oxide (AZO) glass I had purchased earlier, to verify that the pinholes were a product of the substrate and not the plating method itself. The resulting film was completely uniform and pinhole-free, so at this point I made a number of solar cells by repeating this process, applying conductive silver paint over the copper oxide layer, and baking the cells for 30 minutes at 120°C to ensure dryness. The largest of these cells (7 square centimeters of active area) can be seen at the top of the page. Under direct sunlight, it produced an open circuit voltage of 320mV and a short circuit current of 0.77mA, or 0.11mA per square centimeter.
While I believe this is a record for homemade solar cells, the power output is still far too low to be useful. Before focusing on optimization however, my next goal will be to resolve the compatibility issue with my homemade conductive glass. The AZO glass works well, but is expensive and in limited supply. If I can make a working cell with my own glass, the only expensive component remaining will be the silver paint, which I also intend to replace with a cheaper alternative. Beyond that point, my experimentation will likely focus on reducing the resistivity of the copper oxide, either by doping or by modification of the plating process. Overall, this type of solar cell seems promising, and with further experimentation I believe I will be able to produce a useful device.