
|
|---|
Thermoelectricity is the phenomenon by which a thermal gradient produces a voltage gradient within a conductor, and of all the conductive elements, bismuth exhibits this effect to the greatest degree. Furthermore, certain additives are capable of modifying this effect in bismuth, most notably antimony (which enhances the voltage) and tin (which reverses its polarity). Tellurium is also useful for this purpose, with bismuth telluride being the foundation of modern thermoelectric generators; however, tellurium itself is exceptionally rare, and its compounds with bismuth are universally brittle and air-sensitive. For this reason I decided to explore various bismuth alloys containing antimony, zinc, tin, and lead, in order to develop a pair of positive and negative thermoelectric materials (a thermocouple) which are thermally and electrically matched, as well as castable, machinable, and efficient.
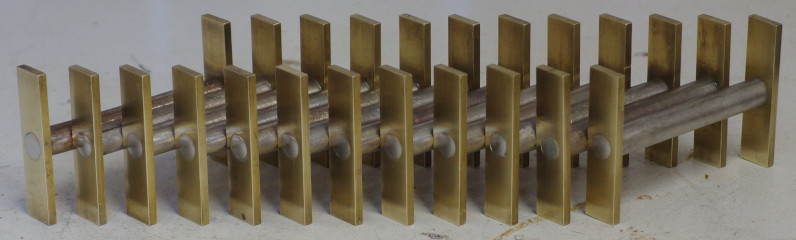
I started by making a cylindrical graphite mold, so that I could cast identical bars of the various alloys to be tested, as well as a rectangular ingot mold for storing excess material. The alloys were then made from raw elements of at least 99% purity, by first melting the bismuth component in an electric furnace, then adding the alloying elements (with antimony being added last) at the alloy's optimal casting temperature (typically 50°C higher than its melting point) while stirring with a graphite rod. Each alloy was then cast into the cylindrical mold, which was held together with a steel clamp and heated to at least 150°C to avoid freezing the casting prematurely. Each bar was then cut to length and machined to fit into brass plates at either end, which serve as thermal and electrical conductors for testing purposes. The bars were then soldered to the plates with eutectic tin-bismuth solder paste, which bonds well to all of the alloys tested, and which flows at a low enough temperature to avoid melting the bars themselves. Finally, the assembled bars were annealed for four hours at 85°C, which is below the melting point of any possible eutectic phases. The completed test samples can be seen at the top of the page, while the ingredients and molds can be seen below.


|
|---|
With the samples prepared, I began by measuring each alloy's thermoelectric coefficient by submerging one end-plate in boiling water and the other in ice water, then measuring the voltage produced across the bar. Ideally this setup would establish a constant temperature differential of 100°C; however, direct readings using a thermocouple probe revealed a difference closer to 75°C, likely due to the poor thermal conductivity of the water as well as radiative losses from the end-plates. For this reason I have described the calculated coefficients as a range rather than a precise value, with the low end being the measured voltage divided by 100°C, and the high end being the same voltage divided by 75°C.
Next, I measured each bar's resistance by using a constant-current power supply to push one amp through the bar, then measuring the voltage produced across it. This method allows for the measurement of very small resistances using an ordinary multimeter, and due to Ohm's law, the measurement in volts is equal to the resistance in ohms. I then multiplied this resistance value by the bar's cross-sectional area (in square meters) and divided it by the bar's length (in meters) to calculate the resistivity of each alloy in ohm-meters. This was done both at ambient temperature (20°C) as well as at the midpoint of the temperature range used above (50°C), in order to observe any temperature-dependency as well as to improve the accuracy of future efficiency calculations.
Having no accurate method to directly measure the thermal conductivity of these alloys, I instead opted to calculate an approximate value using the Wiedemann-Franz law. This law states that the ratio of thermal conductivity to electrical conductivity is relatively constant for all metals; therefore, by multiplying the known values of thermal conductivity and resistivity (the inverse of conductivity) for pure bismuth at ambient temperature (8 W/(m·K) and 1.3x10-6 ohm·m, respectively), and dividing by the resistivity of a given alloy, its thermal conductivity can be estimated. This is admittedly a rough estimate, since bismuth and antimony are semimetals and likely deviate from this law somewhat; however, these results are backed up qualitatively by later tests.
With all of the above values recorded, an estimate of the thermoelectric efficiency can be calculated by simply dividing the electrical power produced at a given temperature differential by the heat transferred to generate it. The simplified formula for this is (VT)²/(RCT), where V is the thermoelectric coefficient (in volts per degree kelvin), T is the temperature differential (in degrees kelvin), R is the resistivity of the alloy (in ohm-meters), and C is its thermal conductivity (in watts per meter-kelvin). Note however that this formula uses the total power generated by the thermoelectric material when short-circuited, producing heat within the material itself. The maximum power which can be delivered to a load (and therefore the maximum output efficiency) is 1/4 of this, which occurs when the load has the same resistance as the the material.
The above values, along with the composition and mechanical characteristics of each alloy, can be seen in the table below. Of particular note is that the alloy compositions are specified in terms of percentages of additives by weight, and that the machinability rating is a qualitative assessment of the cutting action of a lathe tool. Poor machinability indicates that the material has a tendency to fracture rather than cut, while fair machinability indicates that the material can be cut but produces powdery shavings (much like cast iron). Good machinability indicates that the material produces short, ductile chips (much like free-machining brass), which shows that the alloy has some degree of flexibility. For the purpose of readability, I have also color-coded the sections which have certain preferable outcomes, to more easily identify the most useful alloys.
| Composition | Thermoelectric Coefficient @ 0-100°C (10-6 V/K) | Resistivity @ 20°C (10-6 ohm·m) | Resistivity @ 50°C (10-6 ohm·m) | Thermal Conductivity @ 50°C (W/(m·K)) | Thermoelectric Efficiency @ 0-100°C | Casting Temperature | Machinability |
|---|---|---|---|---|---|---|---|
| Bi | (-) 57 - 76 | 1.3 | 1.5 | 6.9 | 3.1 - 5.6% | 300°C | Poor |
| Bi-5Zn | (-) 45 - 60 | 1.0 | 0.8 | 13.0 | 1.9 - 3.5% | 350°C | Good |
| Bi-5Sn | (+) 33 - 44 | 2.9 | 3.2 | 3.3 | 1.0 - 1.8% | 300°C | Good |
| Bi-5Pb | (-) 17 - 23 | 3.2 | 3.3 | 3.2 | 0.3 - 0.5% | 300°C | Good |
| Bi-5Sb | (-) 63 - 84 | 1.4 | 1.8 | 5.8 | 3.8 - 6.8% | 350°C | Fair |
| Bi-5Sb-5Zn | (-) 52 - 69 | 1.8 | 1.8 | 5.8 | 2.6 - 4.6% | 450°C | Good |
| Bi-5Sb-5Sn | (+) 51 - 68 | 2.7 | 2.8 | 3.7 | 2.5 - 4.5% | 350°C | Good |
| Bi-5Sb-5Pb | (-) 60 - 80 | 3.7 | 3.3 | 3.2 | 3.4 - 6.1% | 350°C | Good |
| Bi-10Sb | (-) 64 - 85 | 1.6 | 1.8 | 5.8 | 3.9 - 6.9% | 400°C | Fair |
| Bi-10Sb-5Zn | (-) 59 - 79 | 2.3 | 2.4 | 4.3 | 3.4 - 6.0% | 500°C | Fair |
| Bi-10Sb-5Sn | (+) 47 - 63 | 2.4 | 2.4 | 4.3 | 2.1 - 3.8% | 400°C | Fair |
| Bi-10Sb-5Pb | (-) 30 - 40 | 3.7 | 3.6 | 2.9 | 0.9 - 1.5% | 400°C | Fair |
The above data is complex, but a few patterns can be observed within it. First, the addition of antimony generally improves bismuth alloys, but is most useful at around 5% by weight. Mechanically, it also acts as a hardener and raises the melting point of the alloy. On the other hand, zinc is a poor addition overall; although it does temper the brittleness of bismuth, it also lowers its thermoelectric coefficient significantly, and interferes with additions of antimony by forming zinc antimonide. This side product (though itself a useful thermoelectric material) causes the melt to oxidize rapidly and stick to graphite, while also significantly raising the casting temperature, and is generally a nuisance to deal with. Meanwhile, tin and lead appear to make a perfect pair. Both lower the thermal conductivity of bismuth, improve its machinability and casting characteristics, and produce similar (but opposite-polarity) thermoelectric coefficients with the help of antimony.
Overall, the alloys containing 5% antimony and 5% of either tin or lead (Bi-5Sb-5Sn and Bi-5Sb-5Pb) appear to make the most useful thermocouple, and experimentally they appear to be thermally matched. By standing a bar of each alloy upright on a flat graphite block heated by a hot plate, and using two thermocouple probes to observe the rise in temperature of the opposite ends, I found that the difference in temperature between the alloys never exceeded one degree Celsius. Although this test is fairly qualitative, it directly relates to the circumstance under which the alloys will be used, so I am happy with the results.
Since these alloys can be cast as easily as pewter and machined as easily as brass, it should be relatively straightforward to create thermoelectric generators of any size or shape. The only limitations are the melting points of the alloys and the number of couples required to produce a given voltage, both of which can be adjusted for in the overall design, and at this point I intend to focus on exactly that. My next projects will consist of turning these materials into useful devices, which will generate electricity from various sources of heat without the need for any moving parts, and which can be constructed (and repaired) with ordinary mechanical equipment.